Sds Page Reducing Conditions
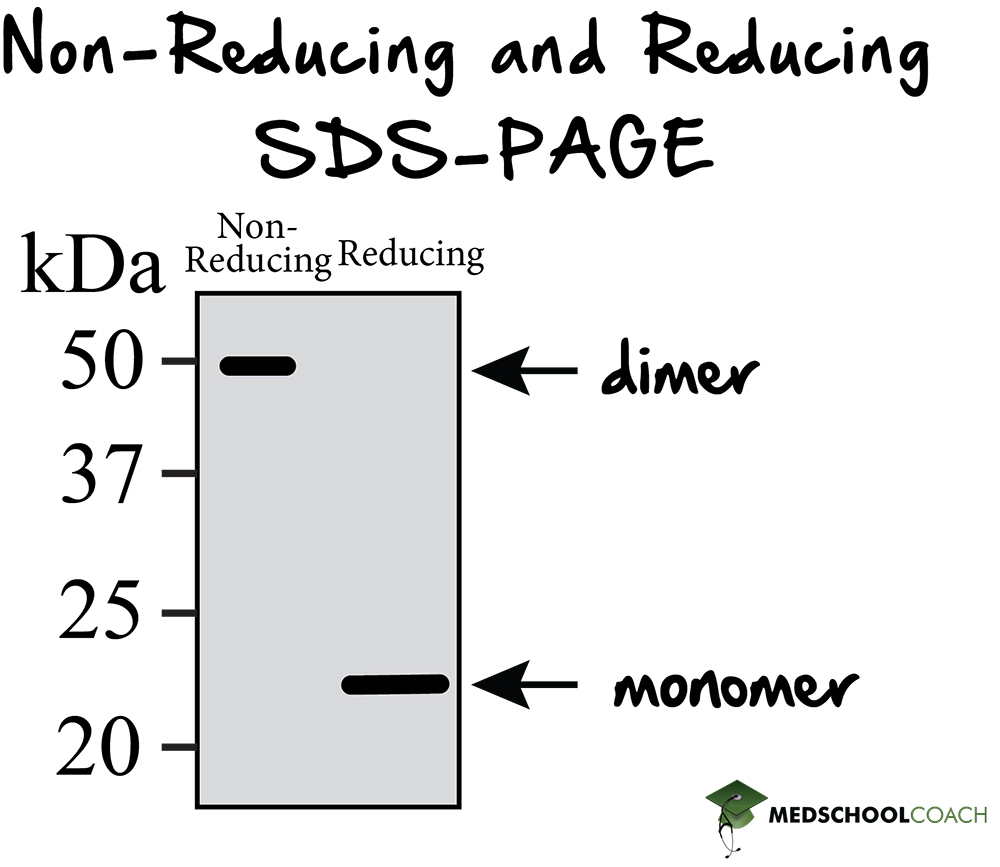
Sds Page Reducing Conditions - A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band.
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band.
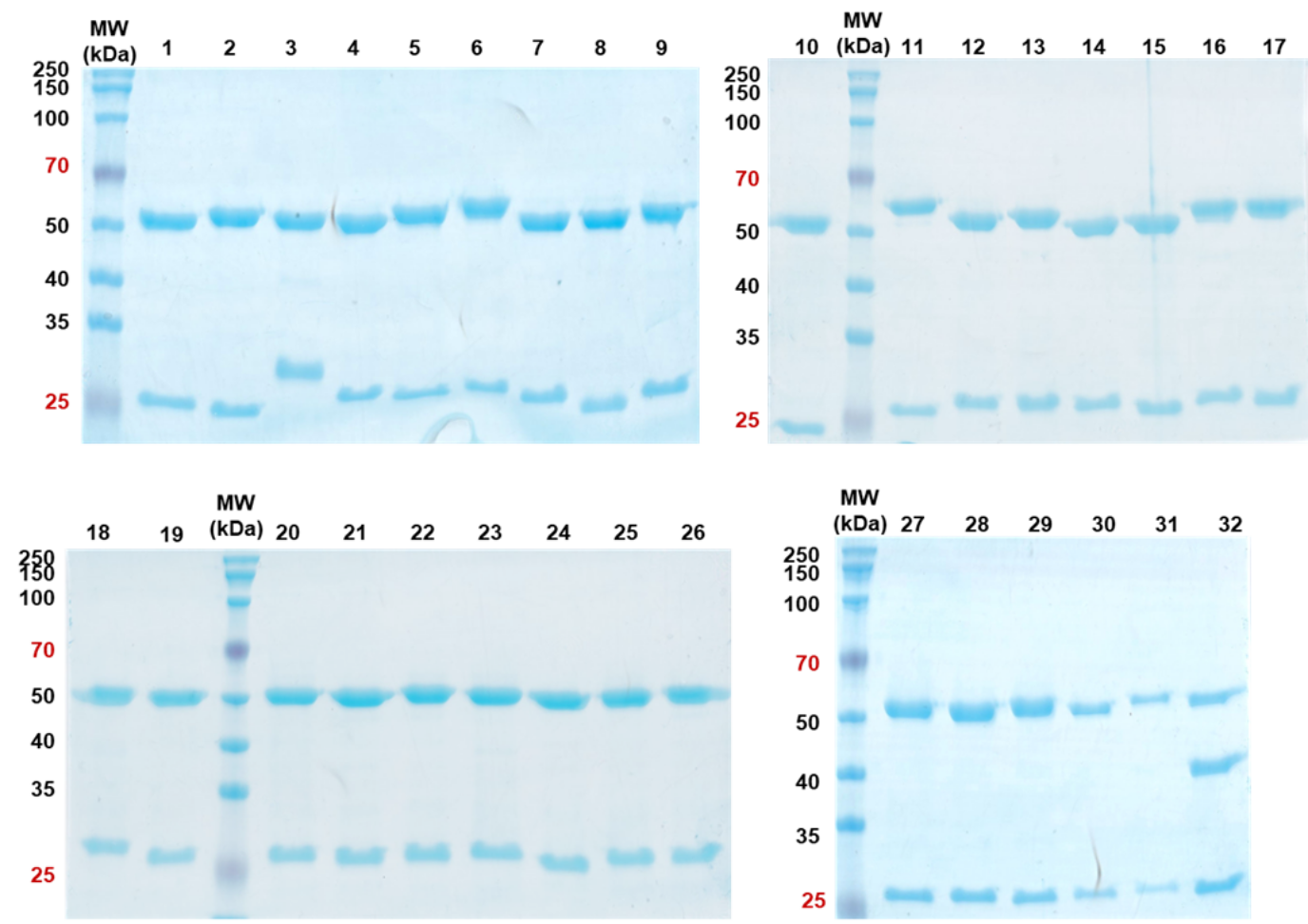
Reducing Sds Page And Non Reducing Sds Page Analysis Of Purified Hprl
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
SDSPAGE showing protein release (reducing conditions), cellular uptake
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
SDSPAGE under reducing (A) and nonreducing conditions (B) of
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
SDSPAGE of IgG and F(ab’) 2 preparations. Protein samples in SDS
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
SDSPAGE and western blot analysis of VHHFc seed extracts. About 32 µg
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
SDS PAGE tutorial Biochemistry Pinterest Chemistry
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band. By heating the sample under denaturing and reducing conditions, proteins become unfolded and.
Reducing SDSPAGE and nonreducing SDSPAGE analysis of purified hPRL
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band.
High Throughput Antibody Production ProteoGenix
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band.
Gel Electrophoresis, PAGE, SDS PAGE MCAT Biochemistry MedSchoolCoach
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
SDS (20)PAGE analysis under reducing conditions of monoclonal IgG
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
If We Had A Heterotrimer, We Would Only See One Band.
By heating the sample under denaturing and reducing conditions, proteins become unfolded and. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.