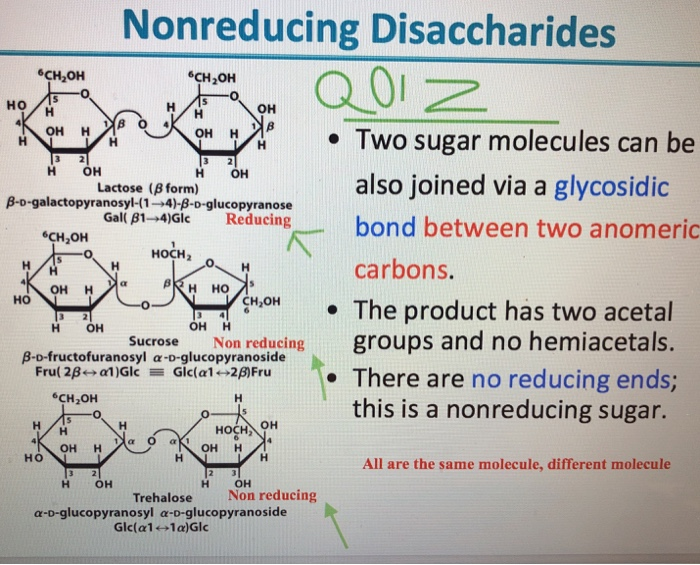
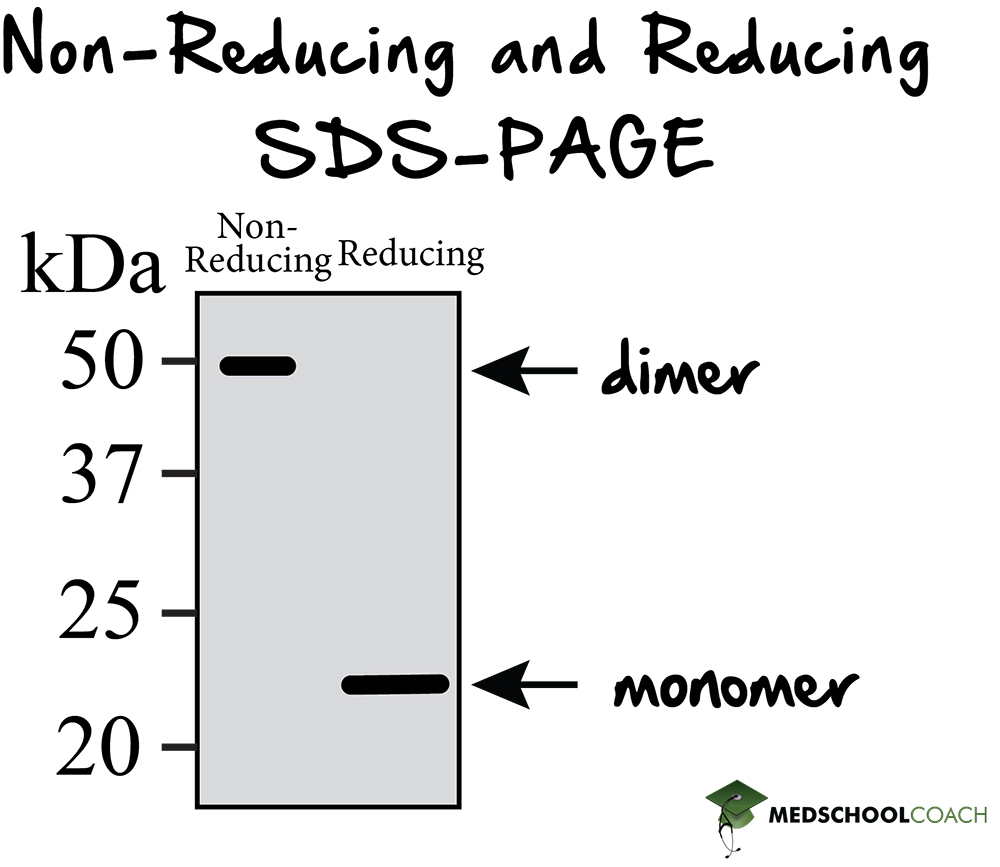
Reducing Vs Non Reducing Sds Page
Reducing Vs Non Reducing Sds Page - If we had a heterotrimer, we would only see one band. So in reducing sds, you add bme or another. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
If we had a heterotrimer, we would only see one band. So in reducing sds, you add bme or another. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. So in reducing sds, you add bme or another.
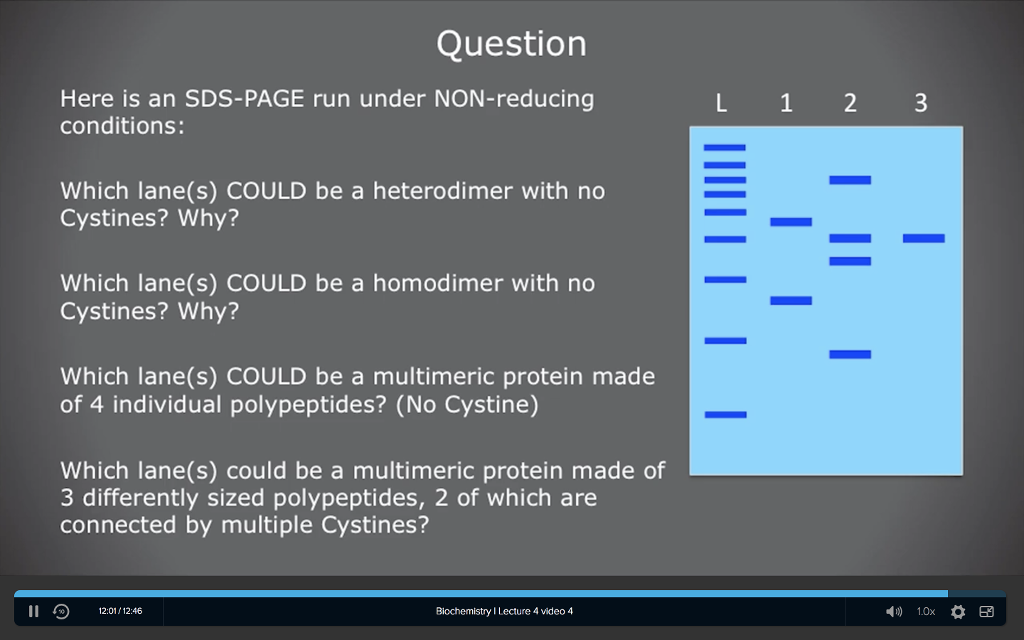
Solved Question Here is an SDSPAGE run under NONreducing
If we had a heterotrimer, we would only see one band. So in reducing sds, you add bme or another. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
HumanKine® human GDNF protein Proteintech
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band. So in reducing sds, you add bme or another.
Reducing vs Non reducing SDS Page [SB B/B 69 Spoiler] r/Mcat
If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. So in reducing sds, you add bme or another.
Separation of low molecular weight oligomers. Nonreducing SDSPAGE
So in reducing sds, you add bme or another. If we had a heterotrimer, we would only see one band. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
Native vs. Nonreducing SDS vs reducing SDS r/Mcat
If we had a heterotrimer, we would only see one band. So in reducing sds, you add bme or another. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not.
Reducing vs. Non Reducing Sugars What's the Difference? Diffesaurus
So in reducing sds, you add bme or another. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
Reducing Sds Page And Non Reducing Sds Page Analysis Of Purified Hprl
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band. So in reducing sds, you add bme or another.
SDSpage reducing vs. nonreducing vs. native Student Doctor Network
A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. So in reducing sds, you add bme or another. If we had a heterotrimer, we would only see one band.
Solved Help please, I don’t understand how to distinguish
So in reducing sds, you add bme or another. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not. If we had a heterotrimer, we would only see one band.
A Reducing Agent Can Break Disulfide Bonds, And For A Majority Of Proteins, This Will Not.
So in reducing sds, you add bme or another. If we had a heterotrimer, we would only see one band.
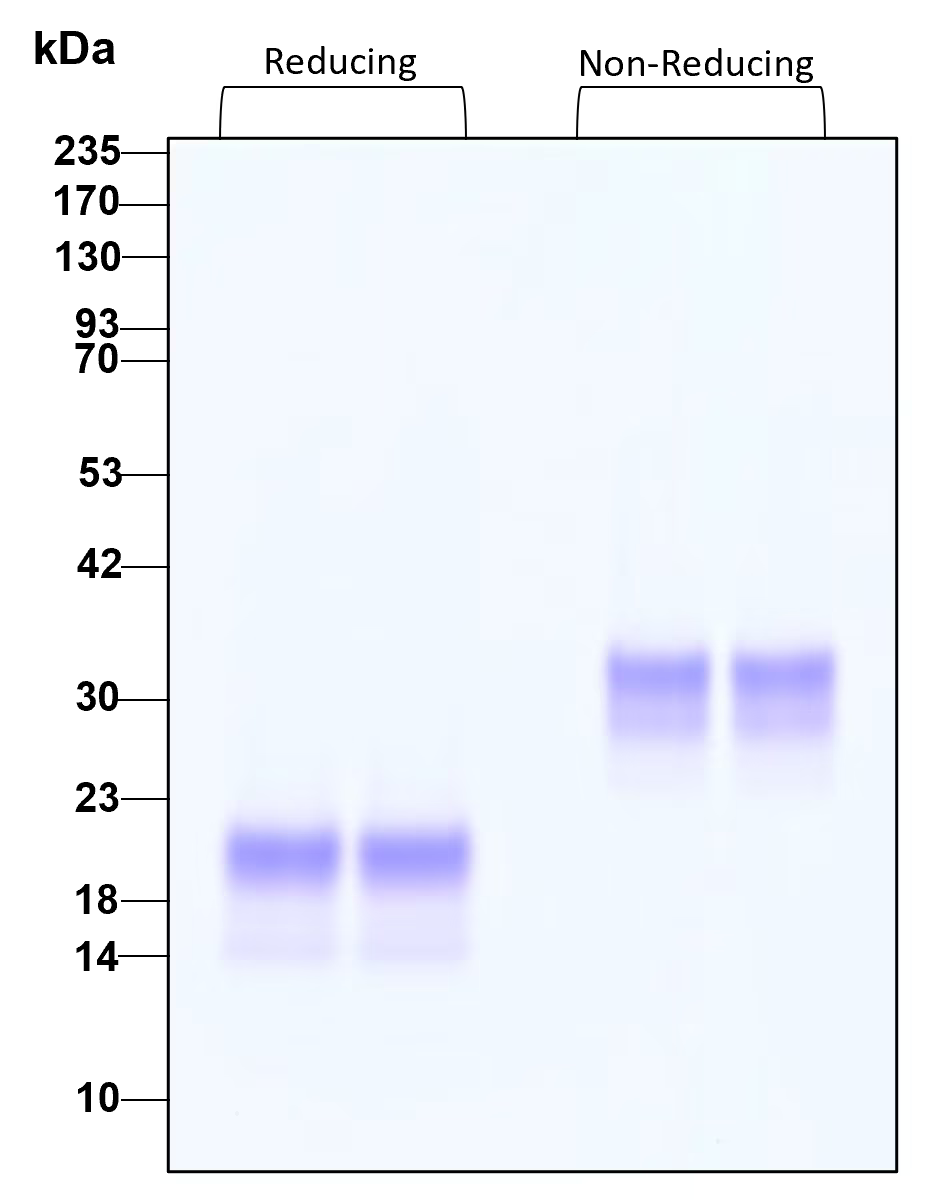
![Reducing vs Non reducing SDS Page [SB B/B 69 Spoiler] r/Mcat](https://preview.redd.it/ipwmbe9hoaa81.png?width=1920&format=png&auto=webp&s=439ec725a25f0a8f4e8fcf204f5769629fe2970a)